Of the Best Examples Which Best Describes Water Cohesion
Surface tension _____causes water to spread out is the ability of water to dissolve a substance creates a thin elastic layer of hydrogen atoms is caused by. Waters cohesive and adhesive properties allow this water strider Gerris sp to stay afloat.

Soil Water Content An Overview Sciencedirect Topics
One of the most common examples is water beading up on a hydrophobic surface.

. The surface tension is due to cohesion water molecules attracting each other. The surface tension formed by cohesion allows it for the light objects to float on water with no sinking. The spherical shape of water is attributed to the surface tension of water.
O Water can move up a 100-foot pine tree from the roots to the leaves. Which answer best describes cohesion. Both cohesion and adhesion Groundwater travels upward through soil from wet to dry areas.
For example dew drops form a dome when filling a container before it is spilling over the sides. When light objects float on water instead of sinking. An example of cohesion would be water molecules sticking.
Adhesion is only found in mixtures and compounds. Cohesion Some insects can float on the waters surface due to high surface tension. This tutorial gives an overview of the nervous.
Water forms into a drop on a leaf and hangs down but it does not drop. O A can of soda bursts when it is placed in the freezer. Droplets of water on a leaf such as in Figure 1.
O Water requires a great deal of heat to reach the point of vaporizing. Cohesion describes waters attraction to itself. Some examples of cohesion are.
Cohesion usually occurs when a liquid comes in contact with a surface. The first two water cohesion examples are due to the. Cohesion is the term for molecules of a substance sticking together.
The cohesive force governs the shape which the liquid mimics. Schmidt National Park Service. Adhesion Water sticks to the surface of a membrane.
One example of adhesion is water climbing up a paper towel that has been dipped into a glass of water and one example of cohesion is rain falling as drops from the sky. O A rock skipping across the surface of a lake. Cohesion is the property of water that allows liquid to be changed into gases.
One water molecule is held to another molecule through covalent bonding. A water drop is composed of water molecules that like to stick together-an example of the property of cohesion. Of the following examples which best demonstrates the property of water cohesion.
Cohesion describes waters _____attraction to other substances attraction to itself ability to dissolve most substances rate of cooling and heating Weegy. A simple example of cohesion in action comes from the water strider below an insect that relies on surface tension to stay afloat on the surface of water. Water molecules all have a positive.
When under tension or pulled from the top as when water on top escapes to the air due to evaporation transpiration the vertical ascent is even. Cohesive forces that occur in liquid makes intermolecular forces resist separation. Water molecules are what are called dipoles.
They have an electric pole at each end of the molecule with opposite charges because the electrons in the molecule tend to congregate near the oxygen atom and away from. Cohesion definition is - the act or state of sticking together tightly. Cohesion describes waters attraction to itself.
All forms of water rain in a glass etc are examples of cohesion. In the picture of pine needles above the water droplets are stuck to the end of the pine needles-an example of the property of adhesion. Image of a water strider bug walking on the surface of water.
Cohesion is a term used to coin the attraction between molecules of the. In another example insects such as the water strider use the surface tension of water to stay afloat on the surface layer of water and even mate there. During adhesion water is attracted to other substances causing the positive and negative molecules of the water to be attracted to the paper.
The adhesion of water is best explained as the ability of water to stick to other surfaces through the creation of weak surface bonds. The cohesion and adhesion properties of water as well as its surface tension make possible its vertical ascent in a narrow glass tube in an unbroken column of fluid. Water is polar which allows molecules to bond together and stick to each other.
This is possible thanks to the surface tension of the water. This is called capillarity. Because the water molecules are more strongly attracted to each other to that of other molecules they form droplets on the surfaces.
2 2 Water Concepts Of Biology 1st Canadian Edition
2 2 Water Concepts Of Biology 1st Canadian Edition

Cohesion And Adhesion Water Properties Examples Expii

Properties Of Water Fill In Interactive Notebook Page Interactive Notebooks Teaching Biology Chemistry Help

Cohesion Versus Friction In Controlling The Long Term Strength Of A Self Healing Experimental Fault Weiss 2016 Journal Of Geophysical Research Solid Earth Wiley Online Library

Cohesion And Adhesion Water Properties Examples Expii

Cohesion And Adhesion Water Properties Examples Expii

Cohesion And Adhesion Water Properties Examples Expii

Cohesion And Adhesion Water Properties Examples Expii

A Linear Relationship Between Liquid Production And Oil Field Subsidence

Chemistry Tutorial Hydrogen Bond Water Molecule Molecules

Chemistry Tutorial Hydrogen Bond Water Molecule Molecules

Molecules Of Life Powerpoint Molecules Polarity Of Water Life
2 2 Water Concepts Of Biology 1st Canadian Edition

Cohesion And Adhesion Water Properties Examples Expii
2 2 Water Concepts Of Biology 1st Canadian Edition
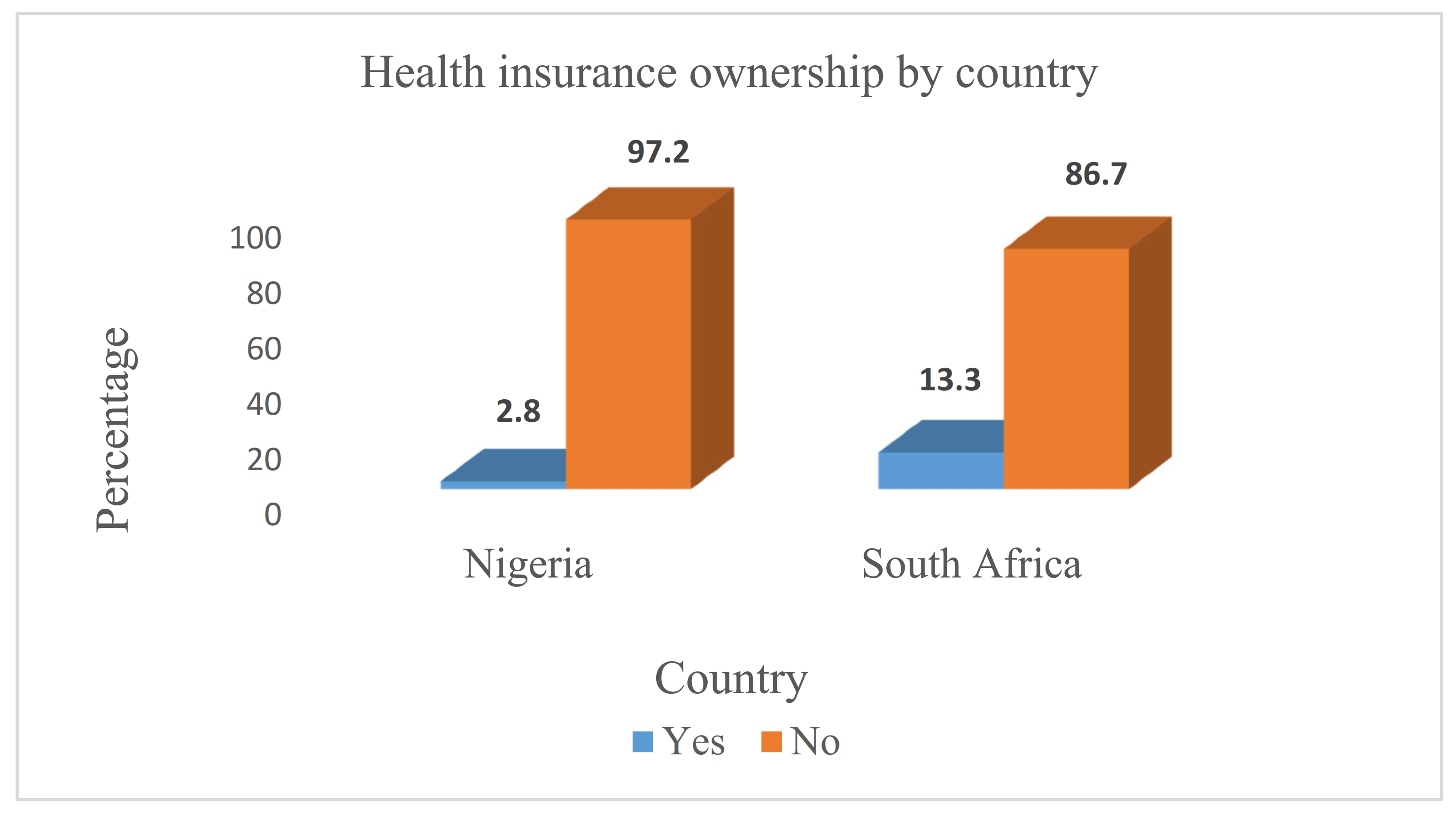
Ijerph Free Full Text A Comparative Cross Sectional Study Of The Prevalence And Determinants Of Health Insurance Coverage In Nigeria And South Africa A Multi Country Analysis Of Demographic Health Surveys Html
Comments
Post a Comment