Can a Fluorine Atom Ever Form a Nonpolar Covalent Bond
Given that it has the highest electronegativity can a fluorine atom ever from a non-polar covalent bond. 15 - What drives an atom to form a covalent bond.

Is Glucose Polar Or Nonpolar C6h12o6 How To Find Out Molecules Math
15 - How are metallic bonds similar to ionic bonds.

. Can magnesium form a covalent bond. Can a fluorine atom ever form a nonpolar covalent bond. Answer 1 of 3.
When two fluorine atoms come together they each share one of. Choose the compound below that contains at least one polar covalent bond but is nonpolar a. You can model this as a result of having empty d-orbitals but the interesting thing is that computational chemistry shows no involvement by valence d.
Types of Bonds can be predicted by calculating the difference in electronegativity. Compare the bond in fluorine F2 with the bond in hydrogen fluoride HF in Figure 25. Since fluorine is in group 17 of the periodic table which means it has 7 valence electrons it only needs one more to complete its octet 8 electrons in its valence shell.
A nonpolar covalent bond implies that both electrons that form the bond between the fluorine atoms are shared equally. Yes in F2 in which the eletronegativity difference between the two fluorine atoms is 0. If Electronegativity difference is Less than 04 then it is Non Polar Covalent Between 04 and 17 then it is Polar Covalent Greater than 17 then it is Ionic.
Electra negativity cannot flooring Adam ever for me non polar Kobe Alan Bond. When two fluorine atoms come together they each share one of their 7 valence electrons to form a nonpolar covalent bond. Since fluorine is in group 17 of the periodic table which means it has 7 valence electrons it only needs one more to complete its octet - 8 electrons in its valence shell.
What about F2 F bonding with itself. Why does the distance between two nuclei in a covalent bond vary. What elements form nonpolar covalent bonds.
Single or multiple bonds between carbon atoms are nonpolar. A nitrogen atom can fill its octet by sharing three electrons with another nitrogen atom forming three covalent bonds a so-called triple bond. Will lithium ever form covalent bonds.
For Be and F. The whole compounds oh are not non polar when all of their bonds are non polar. In polar covalent bonds the electrons are shared unequally as one atom exerts a stronger force of.
Compounds are not just non-polar when all of their bonds are non-polar but when a molecule has no overall dipole moment. As I already stated above a nonpolar covalent bond occurs when there is equal sharing between two atoms of electrons in. Covalent bonds form when electrons are shared between atoms and are attracted by the nuclei of both atoms.
1 Magnesium and chlorine form an ionic bond. In hydrogen fluoride fluorine attracts electrons more strongly than hydrogen does so the bond formed is polar. 15 - Write the electron-dot structure for the covalent.
A nonpolar covalent bond implies that both electrons that form the bond between the fluorine atoms are shared equally. The bond present in F2 molecule is formed by sharing of electrons hence it is a covalent bond. Any covalent bond between atoms of different elements is a polar bond but the degree of polarity varies widely.
So the difference in EN is 40 - 40 0 and it is a covalent bond 100 covalent. When electrons are shared equally they spend the same amount of time on both atoms that form the bond that is why the. Because sulfur has six valence electrons and room around itself for six bonds.
15 - Atoms of nonmetallic elements form covalent bonds. Given that it has the highest electronegativity can a flouride atom ever form a nonpolar. Yes a fluorine atom will form a nonpolar covalent bond with another fluorine atom to form the F2 molecule.
A covalent bond that has an equal sharing of electrons and the electronegativity difference is zero is called a nonpolar covalent bond. Given that it has the highest electronegativity can a fluorine atom ever form a nonpolar covalent bond. Since it is the outermost electrons that engage in bonding the length of the bond depends on electrons in atom A and atom B coming close enough together that their outermost orbitals overlap.
Therefore the nuclear attraction is strong compared to the rest of the alkali metals which leads to a greater polarising power in Li ion and the formation of covalent bonds. ICl3 b SeBr4 c. Some bonds between different elements are only minimally polar while others are strongly polar.
In pure covalent bonds the electrons are shared equally. Ionic bonds can be considered the ultimate in polarity with electrons being transferred rather than shared. Polar Covalent Bond When the electrons spend more time around the more non-metallic atom the sharing of the electron pair becomes unequal and results in the formation of polar covalent bonds.
When electrons are shared equally they spend the same amount of time on both atoms that form the bond that is why the. But when a compounds has no for all di poll moments on an example of this will be the Compound C four where there is a DYP whole moment between I Harbin and Florian so that would be. 15 - Two fluorine atoms join together to form a.
When two fluorine atoms come together they each share one of their 7 valence electrons to form a nonpolar covalent bond. Does F2 have nonpolar covalent bonds. Figure 25Nonpolar and Polar Bonds Fluorine forms a nonpolar bond with another fluorine atom.
Can a fluorine atom ever form a nonpolar covalent bond. The atomic and ionic radii of Li are very small. Given that it has highest electronegativy can a flourine atom ever form a nonpolar covalent bonnd.

How To Draw If3 Lewis Structure Science Education And Tutorials

Best Overview Is Ch3f Polar Or Nonpolar Science Education And Tutorials

Lecture 3 Polar And Non Polar Covalent Bonds Dr A K M Shafiqul Islam Ppt Download

Is Hf Hydrogen Fluoride Polar Or Nonpolar
Is Sf4 Polar Or Nonpolar What S Insight
Covalent Bonding In Nature Only The Noble Gas Elements Exist As Uncombined Atoms They Are Monoatomic Consist Of Single Atoms All Other Elements Need Ppt Download

Lesson Explainer Electronegativity Nagwa

Notes 5 3 Covalent Bonds Covalent Bond A Force That Bonds Two Atoms Together By A Sharing Of Electrons Each Pair Of Shared Electrons Creates Ppt Download

Fluorine Lewis Dot Structure Drawing Several Compounds And Detailed Explanationstitle Lambda Geeks

Is Of2 Polar Or Nonpolar Oxygen Difluoride Oxygen Molecules Polar

Covalent Bonds How Covalent Bonds Form Atoms Can Become More Stable By Sharing Electrons The Chemical Bond Formed When Two Atoms Share Electrons Is Ppt Download

Science Coverage Is Cf2cl2 Polar Or Nonpolar Molecular Geometry Covalent Bonding Solubility
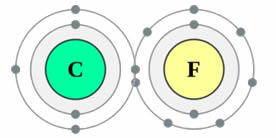
Science Skool Electronegativity And Polarity
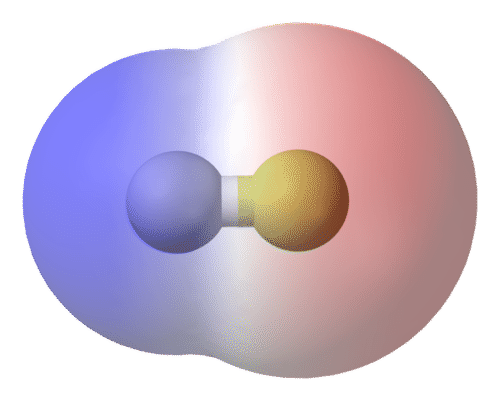
Polarity In Chemical Bonds Ck 12 Foundation

Science Coverage Is No2 Polar Or Nonpolar Electron Affinity Covalent Bonding Molecular Geometry

Is Of2 Polar Or Nonpolar Oxygen Difluoride Oxygen Molecules Polar

Covalent Bonds How Covalent Bonds Form Atoms Can Become More Stable By Sharing Electrons The Chemical Bond Formed When Two Atoms Share Electrons Is Ppt Download
Polar And Nonpolar Covalent Bonds Characteristics Differences

Comments
Post a Comment